PRIMARY CONTAINER & DEVICE SELECTION AND EVALUATION
Timeline
Development activities were completed in 14 months after contract was signed.
Benefit
Company successfully launched their first product in a prefilled pen with no prior pharmaceutical or device experience.
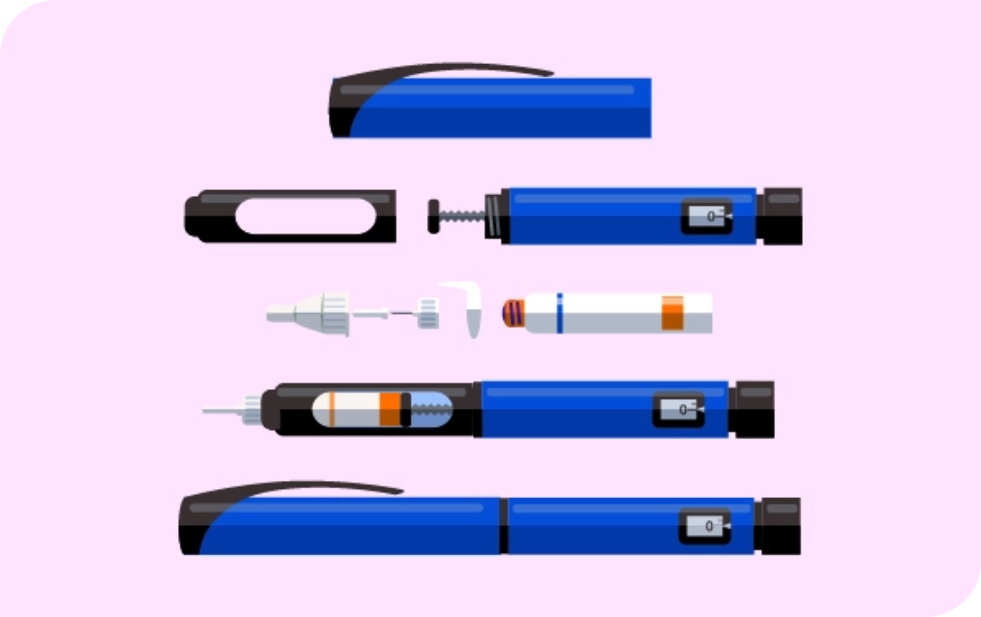
ZebraSci led the following development activities:
- Primary container and device selection consultation
- Container closure integrity testing
- Primary container & device test method development, functional testing, and documentation
- Silicone distribution & functional stability testing
- Final product lot release testing & established market complaint management program