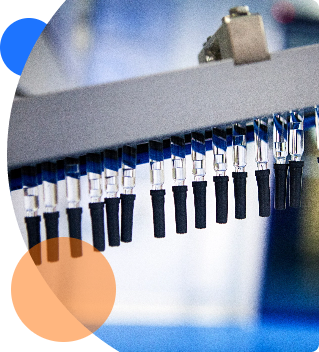
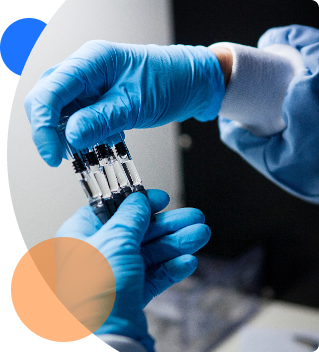
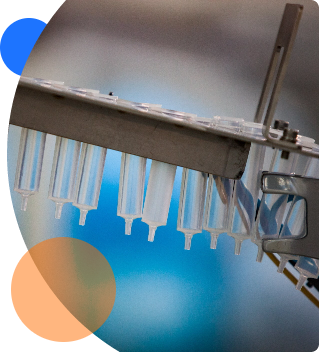
ZebraSci provides our clients with access to our GMP production filling, assembly and labeling not-for-human-use (NFHU). Our years of experience have enabled us to support clients with some of their most complex challenges.
Our expertise includes but is not limited to:
- ISO 7 clean environment
- Bench top single filling station and semi-automated dual filling station
- Stopper setting equipment from Groninger
- Manual and semi-automated device assembly
- Documentation of GMP records for manufacturing traceability
- Isolators able to be used on equipment
- Vacuum and mechanical plunger placement capable processes
- Controlled silicone quantity and distribution applications available for design space testing
- WFI or viscosity matching fill/finish to support equipment validation or device testing
ZebraSci has identified a need in the market to pack and assemble combination products with high customization and small batch sizes. To answer this need in the market, ZebraSci has developed for human use packaging and device assembly services. ZebraSci has the in-process controls, lot release testing, and complaint handling programs to support these services.
Primary Packaging
- Vials
- Cartridges
- Syringes
Device Assembly
- Autoinjectors
- Pen injectors
- On body devices
Tertiary Packaging
- Labeling for parenteral and injectables
- Cartoning
- Kitting