About us
ZebraSci was founded in 2009 as a company dedicated to developing novel inspection technologies to ensure quality in parenteral packaging and combination products. Over the years, we’ve grown substantially by concentrating on technical expertise, customer service and a strict adherence to regulatory compliance. As an independent combination testing and expert advisory platform our goal is to challenge the limits and cultivate unique solutions for our customers. We also leverage the knowledge from our past and the structure of our parent company to expand our capabilities. This has allowed us to continue to test products outside of the BD portfolio while also giving clients an opportunity to gain access to both product and service needs. Our goal is to challenge the limits of this industry and cultivate unique solutions for our customers.

Zebrasci timeline

Founded in 2009 as an equipment company developing a novel technique to inspect silicone oil distribution in primary containers

ZebraSci receives first orders for Flex System
ZebraSci opens company office in Temecula, CA
ZebraSci expands portfolio with Hydro, Flex HD, Flex P, Flex D

ZebraSci machine shop established
ZebraSci pivots into a combination product testing company providing services to life science companies
Investments made into testing equipment and GMP quality systems to support laboratory testing

Clean room to support fill/finish & device assembly built in CA
ZebraSci expands operations to Branchburg, NJ
Combination product development services company:
- Engineering fill/finish & device assembly
- Container and device development
- Incoming inspection of containers and devices
- Final lot release testing
- Complaint investigation & root cause analysis

ZebraSci acquired by BD


New ZebraSci branding
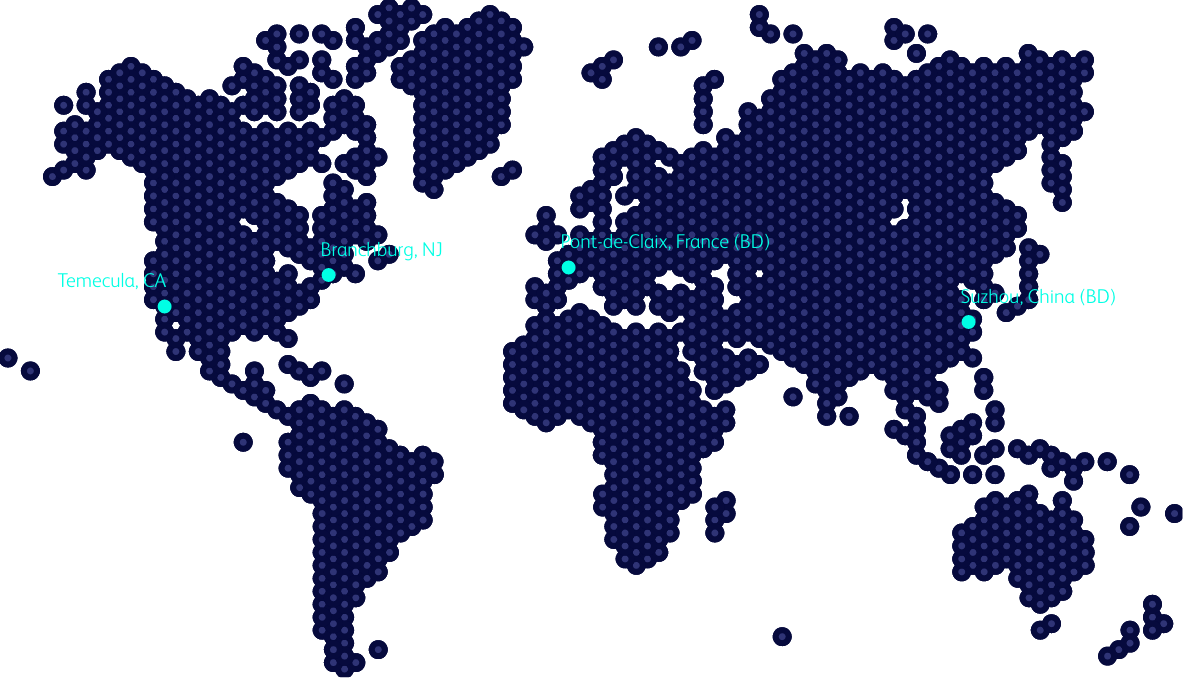
Global presence
ZebraSci is available globally, and we have assisted clients in commercializing numerous combination products in multiple geographies over the last decade. We validate methods on our equipment to match our individual client testing requirements, as needed.
Quality
We have developed and maintain a robust quality management system to demonstrate our commitment to consistently provide inspection products and laboratory services that adhere to cGMP FDA regulations, ISO 17025 and ISO 13485 standards, and customer Quality System requirements.

Meeting your needs
ZebraSci can work with multiple medical device, pharmaceutical, and biotechnology companies for their combination product needs. Client’s partner with us to resolve technical issues, support “time to market” and assist with the development of safe and effective products using our unique approach.
