Drug delivery device selection and evaluation
Select the right components or optimize your drug delivery device to an existing container.
One of the many variables that contribute to drug delivery device reliability is the primary container itself. We have experience with multiple containers and components from many manufacturers, we can assist you with selecting the right components or optimizing your device to an existing container.
Customers can rely on our expertise and experience to thoroughly assess the performance characteristics of their device.

If you need more information, contact us
Request Consultation
What is your request?
VIEW CAPABILITIES
Container critical dimensions (detection limit down to 5 microns or less)
Container critical dimensions (detection limit down to 5 microns or less)
ZebraSci has the measurement system capabilities to quantify critical container dimensions to ensure they meet intended specifications and function properly when assembled together (tolerance stack-up analysis)
- Primary container dimensions
- Plunger stopper dimensions
- Needle inner diameter dimension
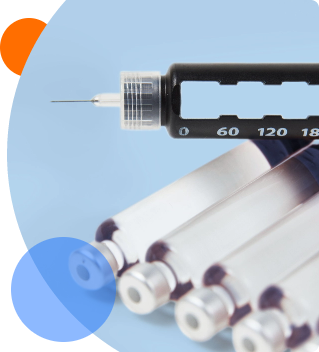
Pre-filled syringe testing
Pre-filled syringe testing
- Rigid needle shield removal force – measures the force required to pull off the needle shield to expose the needle for injection use
- Needle Safety Device (NSD) Activation Force – measures the force required to activate the needle-stick safety feature of the NSD
- NSD separation force – measures the force required to remove NSD components, such as the NSD body and the finger flange
- Break-Loose and Glide Force (BLGF) – Measures the force required to initiate stopper movement (Break-Loose force) and the force required to sustain stopper movement to the end of injection (glide force)
- Deliverable volume – measures the volume of the delivered dose to ensure that the required amount of drug product is delivered
- Syringe flange/tip breakage resistance – measures the force required to break the syringe flange or tip and the resistance to breakage
- Leakage at stopper – pressurizes a filled and stoppered syringe to confirm that liquid does not ingress into the stopper ribs and confirms the system’s resistance to unintended overpressurization by pressurizing
- Syringe fitment with sharps container/carton/refrigerator – confirms that syringe dimensions fits with sharps disposal containers on the market, primary packaging, and refrigeration needs
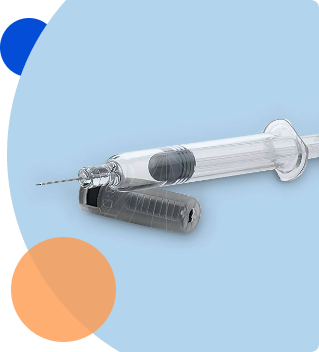
Pen injector testing
Pen injector testing
- Needle attachment/detachment torque – measures the torque required to attach and remove a needle from pen injector
- Dose accuracy (including last dose accuracy) – measures the delivered dose from a pen injector to ensure that it delivers its intended dose. Testing is performed following requirements listed in ISO 11608-1
- Total deliverable volume – measures the total volume of drug in the cartridge
- Pen disassembly – method to disassemble pen injector to non-destructively remove cartridge for additional testing
- Resealability – measures cartridge septum resealability per ISO 11608-3
- Coring – measures cartridge septum coring per ISO 11608-3
- Cartridge Break-Loose and glide force – measures the force required to initiate & sustain plunger movement per ISO 11608-3
- Cartridge CCI – high voltage leak detection and helium leak detection are preferred methods for cartridge CCI testing
- Leakage after pressurization – cartridge will be pressurized and then observed for signs of leakage at the stopper and crimp per ISO 11608-3, by observing the pressurized cartridge
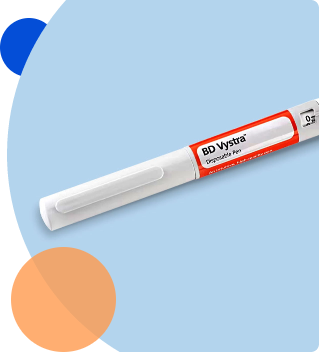
Autoinjector testing
Autoinjector (AI) testing
- Injection time – measure the time to complete a full injection from the device
- Deliverable volume – measures the volume of the delivered dose to ensure that the required amount of drug product is delivered
- Needle injection depth – measures needle penetration depth of AI during intended use
- Cap removal force – measures the force required to remove the AI cap prior to injection
- AI activation force – measures the force required to initiate an injection during normal use
- AI lock-out override force – measures the force required to override the lock-out mechanism for AI devices
- AI fitment with sharps container/carton/refrigerator – confirms that AI dimensions fits with sharps disposal containers on the market, primary packaging, and refrigeration needs

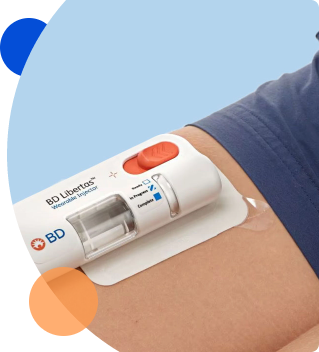
On-body/wearable device testing
On-body/wearable device testing
- Deliverable volume/dose accuracy: the main objective of this test is to measure and verify the actual volume of a drug product delivered by an on-body injector. This is a critical evaluation conducted to assess the ability to accurately deliver the intended dose
- Injection time: this test evaluates the duration it takes for the on-body injector to deliver the drug product into the patient’s body. This test is crucial to assess the time required for the device to deliver the target dose
- Needle injection depth – measures needle penetration depth of on-body injector during intended use
- Needle shield/cap removal force – measures the force required to remove the on-body cap prior to injection
- Button push force – measures the force required to initiate an injection during normal use
- ZebraSci offers a variety of methods to assess container closure integrity. We recommend a combination of Helium and High Voltage Leak Detection for on-body injectors. CCI testing is essential to verify that the container closure system effectively prevents any leakage or contamination of the drug product
Additional tests (device indicators):
- Break-Loose Glide Force: this test can be done to assess the required forces needed to initiate the gliding of injections. This test assesses the ease of activation for the device
- Unintended volume release: this test evaluates the unintended release of drug product from the injector before or after the intended purpose
- End of dose completion: this test is performed to verify that on-body injectors successfully administer the entire dose without any potential interruptions
- Any other test applicable to cartridge assessment (e.g. silicone distribution analysis) will apply to on-body injectors
- Adhesive peel testing: this test measures the force required to remove peels or layers so the patient can easily access the adhesive pad to attach to the body
Other testing
Other testing
- IV Bags and flexible packaging
- Pumps
- If a you are interested in a test method that is not found on our offerings list, we also have capabilities to create new methods to meet your needs
