Common considerations for packaging and shipping vaccines
There is a lot of information circulating about the various COVID-19 vaccines and the logistical challenges certain pharmaceutical companies face due to their vaccine’s temperature storage requirements.

As many people may know, the -70°C temperature requirements for the Pfizer vaccine is an extremely low temperature for storage requirements. In fact, it is so low that we rarely experience this temperature in real life (-70°C is colder than the Winters in Antarctica). This information can cause fear in people who may already be hesitant about receiving a COVID vaccine. If a temperature excursion would cause the efficacy of those doses to diminish, is it safe to get the vaccine? Thankfully, the distribution of vaccines follows a tried-and-true standard and there is a solution for when temperature excursions occur, so the public does not need to worry about receiving a ‘bad’ vaccine. We will touch on that later in the blog.
For this week’s blog about the most common considerations for packaging and shipping for vaccines, ZebraSci and Packaging Compliance Labs teamed up to further explore common considerations for packaging and shipping validation.
Storage and distribution temperature requirements
Temperature is one of the most important considerations for drug products (vaccines and biologics) because the efficacy of the drug relies on the temperature staying within its “sweet spot”. This sweet spot varies depending on what the storage requirement is, and a drug’s storage temperature requirement is determined by stability testing.
After the stability of the vaccine has been evaluated, one of the following recommendations for storage temperature is made:
1. What is typical for distribution and storage temperature?
- Between 2 and 8°C (refrigerator)
- Between -15 and -50°C (freezer)
- Below -50°C (deep freezer)
For the most part, the desired average refrigerator vaccine storage temperature of 5°C is typical for distribution and storage temperature for a vaccine.
2. Do Lyophilized vaccines require the same kind of temperature control that liquid vaccines do?
Lyophilized vaccine powder is simply a freeze-dried version of their liquid form. The process of freeze-drying the vaccine turns it into a dry disc or powder. When vaccines are in their lyophilized form, they should be stored in refrigerated conditions. Other types of lyophilized drug products (non-vaccines) can be stored at room temperature. So, the question is, couldn’t the pharmaceutical industry simply freeze-dry their vaccine to bypass the frozen cold chain requirements and challenges? While it might seem like that is a simple solution, it is not a typical practice.
Typically, when a vaccine is lyophilized it is because the data they receive during stability testing tells them that the vaccine is not stable in liquid form and requires being mixed prior to its use.
While lyophilization helps bypass some challenges that come along with the vaccine cold chain requirements, it creates challenges for those administering the vaccine. Since it needs to be mixed with a liquid prior to use it creates more work and more room for error for the end-user (ie: a pharmacist or nurse administering the vaccine). As we all know, when steps are added to a process that needs to be repeated and reproduced on mass levels, there is a greater opportunity for error.
So, we know that keeping within the range of temperature is important, now how do we track that?
Distribution temperature monitoring
Whether a pharmaceutical manufacturer is using passive or active cold chain packaging (more on this in the next section), when shipping a drug product, it is required to monitor the thermal shipper for the duration of distribution.
Temperature monitors are placed in the interior of the cold chain qualified shipper which tracks the temperature for the period the shipment is in distribution. Once the shipment arrives at its end location, the data from the temperature monitor is downloaded to reveal whether there were temperature excursions during distribution.
If there were no excursions during transit, then life’s good and we can celebrate! If there were excursions, some further investigation is required. Temperature excursions beyond the acceptable range require a scientifically sound evaluation with documented technical justification to determine if the integrity of the drug product has not been affected.
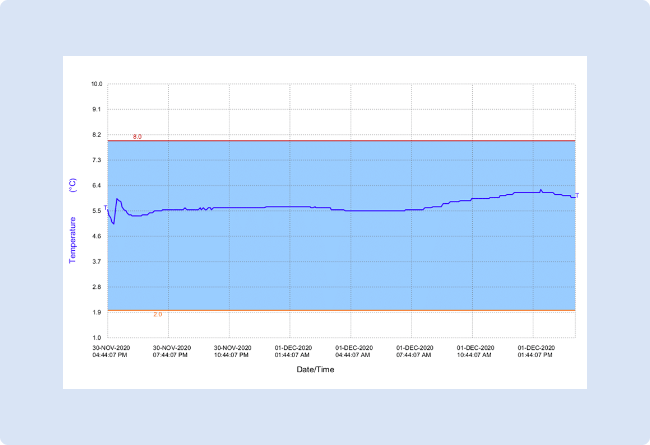
The image below is an example of data downloaded from a temperature tale where the temperature has stayed within the allowable range.
Shipping method
How vaccines are packaged and shipped depends on a handful of things. Temperature requirements, the storage capabilities of its shipping destination, and the quantity of how many vaccines are being shipped are just a handful of considerations.
But the two options for shipping vaccines are choosing a logistics provider or using cold chain qualified packaging.
1. Cold chain logistics provider
Cold chain logistics providers use refrigerated transportation methods (shipping trucks or airplanes) that have been qualified to ensure compliance to temperature requirements. Utilizing a logistics provider usually requires large quantities of vaccines being shipped.
The kind of packaging that would be paired with a cold chain logistics provider is called “active packaging”. When mechanical and electrical means coupled with thermostatic control is used to keep the packaging refrigerated, this is considered an “active” system.
Knowing the shipping rules & regulations
Shipping vaccines within the same country the drug is manufactured is one thing. Shipping vaccines to a country that has restrictions on what kinds of drugs can be shipped is another story. Regulations regarding medical shipments vary from country to country so knowing your supply chain is important. If you know where the drug will be sold you can plan accordingly.
While this blog merely scratches the surface of what is required for vaccines to be packaged and distributed, we hope it answers some questions folks might be having! Please contact ZebraSci or Packaging Compliance Labs if you have any questions or would like to have a more detailed discussion.
It is time for your team to hop on board with this easy-to-use and simple Salesforce feature aimed to boost productivity and efficiency.
Transform Your Sales Process With Salesforce and Syspro.